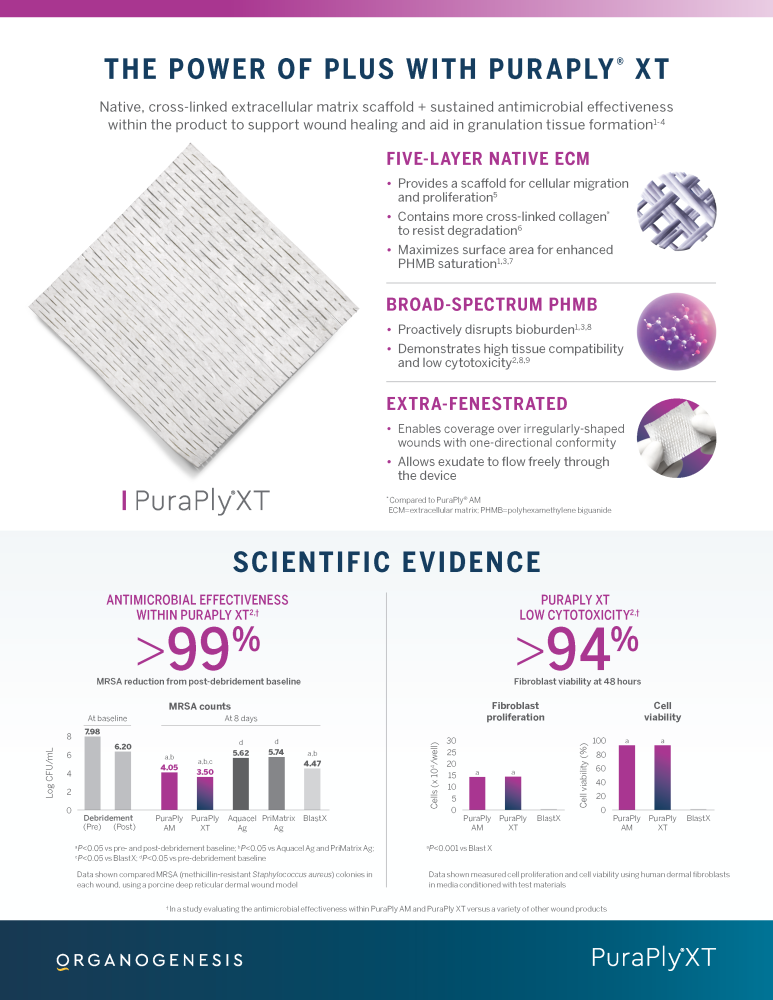
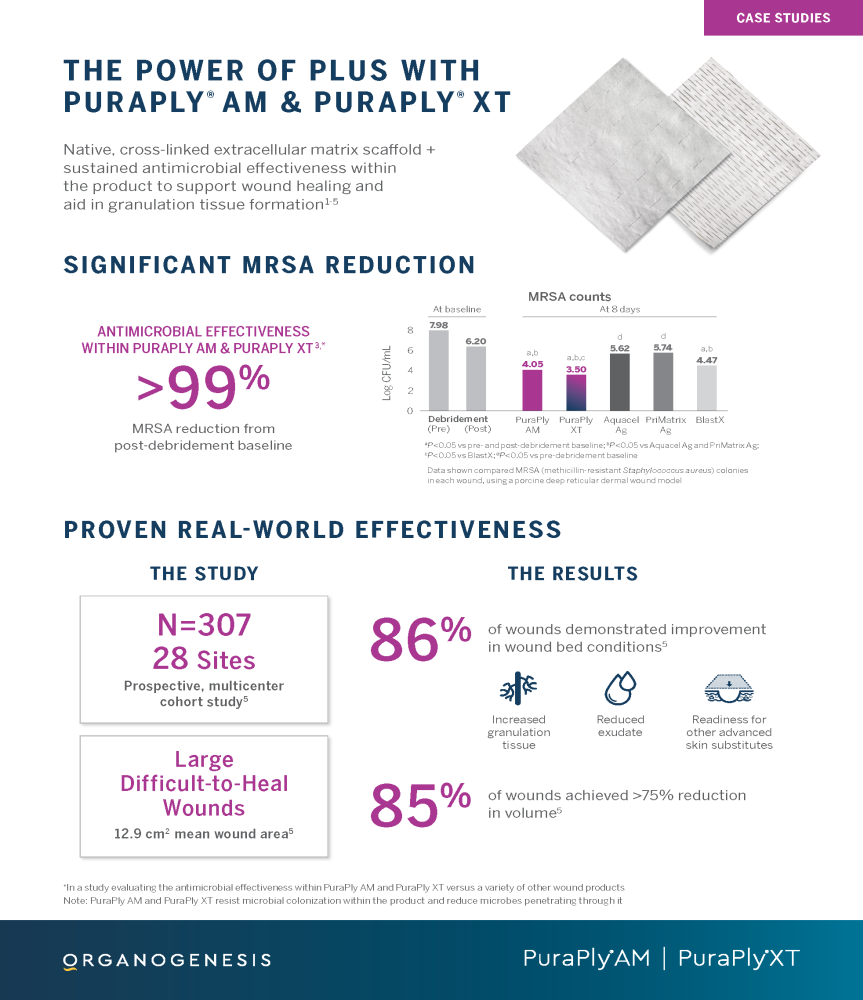
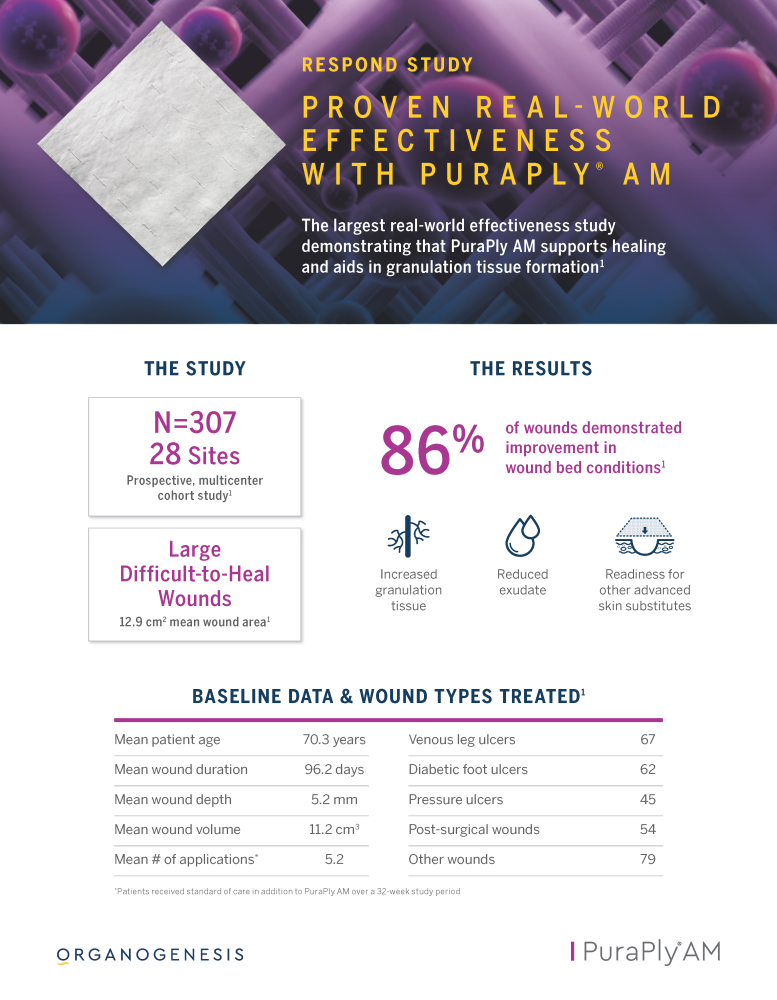
PuraPly® AM and PuraPly® XT
PuraPly® AM and PuraPly® XT are a unique combination of native, cross-linked ECM scaffold plus broad-spectrum PHMB antimicrobial. Intended for the management of acute and chronic wounds including partial- and full-thickness wounds, pressure ulcers, surgical wounds, trauma wounds and venous and diabetic ulcers.

Organogenesis
Organogenesis is a global leader in advanced wound care, offering a comprehensive portfolio of regenerative medicine products capable of supporting patients from early in the wound healing process through to wound closure, regardless of wound type.Benefits
• Provides antimicrobial barrier for controlling bioburden and preventing biofilm reformation
• Produces a sustained antimicrobial barrier effect between weekly debridements
Indications
PuraPly® AM Wound Matrix is intended for the management of wounds and as an effective antimicrobial barrier to resist microbial colonization within the dressing and reduce microbes penetrating through the dressing.
PuraPly® AM Wound Matrix is indicated for the management of: partial- and full-thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic vascular ulcers, tunneled/undermined wounds, surgical wounds (donor sites/grafts, post-Mohs surgery, post-laser surgery, podiatric, wound dehiscence), trauma wounds (abrasions, lacerations, second-degree burns, skin tears) and draining wounds.
Contraindications
This device is derived from a porcine source and should not be used in patients with known sensitivity to porcine material.
The device is not indicated for use in third-degree burns.
Do not use on individuals with a known sensitivity to polyhexamethylenebiguanide hydrochloride (PHMB)
Warnings and Precautions
Do not resterilize. Discard all open and unused portions of PuraPly® AM Wound Matrix.
Device is sterile if the package is dry, unopened and undamaged. Do not use if the package seal is broken.
The device must be used prior to the expiration date.
Discard device if mishandling has caused possible damage or contamination.
PuraPly® AM Wound Matrix should not be applied until excessive exudate, bleeding, acute infection and significant swelling are controlled.
The product can be applied from the onset and for the duration of the wound, weekly or at the discretion of the health care practitioner.
Adverse Effects/Reactions
The following complications are possible with the use of wound dressings. If any of these conditions occur, the device should be removed.
• Worsening infection
• Chronic inflammation (initial application of wound dressings may be associated with transient, mild, localized inflammation)
• Allergic reaction
• Excessive redness, pain, swelling or blistering
Storage Requirements
This device should be stored in a clean, dry location at room temperature.
How Supplied/Sizing
HCPCS Code
Product features
Other features
Mode of Use/Application
Always handle PuraPly® AM Wound Matrix using aseptic technique. Prepare wound area using standard methods to ensure wound is free of debris and necrotic tissue. If necessary, surgically debride the wound to ensure the wound edges contain viable tissue. To apply, cut the dry sheet to the outline of the wound area. If the wound is larger than a single sheet, then multiple sheets may be used. Overlap adjoining sheets to provide coverage of the entire wound. For ease of handling, apply PuraPly® AM Wound Matrix by placing in a dry state over the wound and rehydrate the sheet using sterile saline or other isotonic solution. Place the edge of the sheet in contact with the intact tissue. Smooth PuraPly® AM Wound Matrix into place to ensure the sheet is in contact with the underlying wound bed. The sheet may be fixed to the wound using sutures or other fixation method. The optimum fixation method is determined by wound location, size, depth and user preference. Apply secondary dressings as appropriate for the type and stage of wound.
NOTE: If excess exudate collects under the sheet, small openings can be cut in the sheet to allow the exudates to drain.
Removal & Change Frequency
IMPORTANT: After application, use an appropriate nonadherent secondary dressing to maintain a moist wound environment. The optimum secondary dressing is determined by wound location, size, depth and user preference. Change the secondary dressing as needed to maintain a moist, clean wound area. Frequency of secondary dressing change will be dependent upon volume of exudate produced and type of dressing used. As healing occurs, sections of PuraPly® AM Wound Matrix may gradually peel and may be removed during dressing changes. Do not forcibly remove sections of PuraPly® AM Wound Matrix that may adhere to the wound. Alternatively, the PuraPly® AM Wound Matrix may form into a caramel-colored gel, which can be rinsed away with gentle irrigation. On inspection, if PuraPly® AM Wound Matrix is no longer covering the wound, place an additional piece of PuraPly® AM Wound Matrix over the wound. The wound should be reevaluated on a weekly basis for PuraPly® AM reapplication.
Clinically Tested
Not made with natural rubber latex
No posters match your selected filters. Remove some filtres, or reset them and start again.
Have a product to submit?
Be included in the most comprehensive wound care products directory
and online database.
Learn More