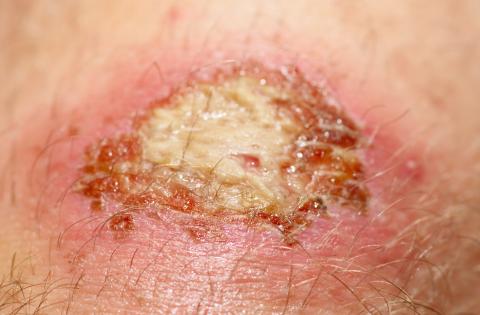
Breaking Down the Stages of Biofilm Formation
January 3, 2025
Biofilms are commonly present in a majority of chronic wounds but are difficult, if not impossible, to identify with the naked eye.1,2 It is important to understand how biofilms form in order to address them properly as part of a wound healing paradigm. In vitro studies have shown that biofilm forms in a rapid multi-step process best addressed as early as possible.1 As the stages progress, it becomes increasingly difficult to eradicate the biofilm.1In vivo studies do not yet exist to confirm this step-wise process, but nonetheless, current observations may provide important insights to clinicians crafting biofilm management plans for patient care. This piece will outline the various stages of biofilm formation as they are currently understood and how this relates to clinical practice.
Stage 1: Reversible Attachment of Microbes
The reversible nature of this stage reinforces the benefit of early biofilm intervention. Planktonic microorganisms are free-floating and typically single cells. However, when multiple organisms converge and embed in a wound, they become immobile, or sessile.1,2 This initial attachment is reversible, and in a noncompromised, well-vascularized host, a robust immune response may fight these organisms. This is not always the case, however, and thus the process of biofilm continues, especially in patients with vascular concerns, tissue necrosis, malnutrition, or medical comorbidities.1 The process of bacteria transitioning from planktonic to sessile can occur within a period of minutes.1
Stage 2: Attachment Becomes Permanent
In this next stage, the sessile microorganisms begin to form colonies. This takes place within 2-4 hours.1
Stage 3: Matrix Secretion Begins
Once the microcolonies begin to converge, the organisms begin the process of secreting extracellular polymeric substances (EPS). EPS make up the protective matrix that contributes to the organisms’ increasing resistance to interventions like antiseptics, disinfectants, etc. The forming biofilm also secretes additional proteins and enzymes that further contribute to enhancing the attachment of the organisms. This stage occurs within 6-12 hours.1
Stage 4: Biocide Tolerance Continues to Increase
Unless a biofilm is disrupted or eradicated, the microcolonies continue to evolve and mature and exhibit increased resistance to biocides. This then cycles into further biofilm development and expansion, all occurring within 2-4 days. The exact time frame can vary based on the type and species of organism, as well as wound conditions.1
Reformation: Biofilm Mitigation is Not a Single Intervention
It is crucial to know that, even when it is disrupted, biofilm is hardy and will quickly bounce back from that intervention.1 Even with appropriate debridement, biofilm will reform as a mature entity in 24-72 hours.1 Therefore, one must remember that the first 24 hours after appropriate debridement is the prime window in which other treatments, such as antimicrobials, may be most effective.1
What Does this Mean for Wound Healing?
There is not clear agreement on exactly how biofilm impairs wound healing, but some explanations cited in the literature include stalling the wound in the inflammatory stage, enhancing abnormal cell phenotypes derived from epidermis and/or dermis, and molecular differences related to matrix metalloproteinases (MMPs) and/or neutrophil elastase (NE).1,2
Options for Intervention
Despite some unknowns about the powerful influence of biofilm on wounds, there are several components to a biofilm-focused therapeutic approach. Debridement is the cornerstone of these plans. Sharp debridement specifically is the gold standard for removing necrotic, devitalized tissue and disrupting biofilm formation.1,2 However, when sharp debridement is not possible or desirable, other types of debridement may be employed. These alternatives, such as ultrasonic, mechanical, or biologic debridement, may disrupt but typically only partially remove biofilm.1
Antimicrobial agents and antibiofilm agents may also be employed as adjunctive interventions. It is important to understand that antibiofilm agents are best defined as those that specifically influence various aspects of biofilm formation, whereas antimicrobials focus on the organisms themselves.1 Examples of mechanisms that comprise antimicrobial options include phage therapy, nanotechnology, inhibitors of population effect, blue light therapy, aptamers, peptide nucleic acids, antimicrobial peptides, and biodegradation enzymes. Examples of mechanisms related to biofilm specifically include pH modification, negative pressure, or surfactants.2 Some options may span both types of agents, including probiotics, mesenchymal stem cells, or honey.2
Conclusion
There is still much to learn about biofilm, but it must be a consideration in any wound healing trajectory. However, breaking down the process of its formation can lead to insights about timing and urgency of intervention. For more on biofilm identification, download this month’s white paper. And for additional insights on intervention, register for this month’s webinar.
Reference
1. Liu Y, Long S, Wang H, Wang Y. Biofilm therapy for chronic wounds. Int Wound J. 2024 Feb;21(2):e14667. doi: 10.1111/iwj.14667. PMID: 38339793; PMCID: PMC10858329.
2. Bjarnsholt T, Eberlein T, Malone M, Schultz G. Management of wound biofilm Made Easy. London: Wounds Int. 2017;8(2).
The views and opinions expressed in this content are solely those of the contributor, and do not represent the views of WoundSource, HMP Global, its affiliates, or subsidiary companies.