Kent Imaging Announces FDA Clearance of SnapshotNIR (KD 205)
July 9, 2024
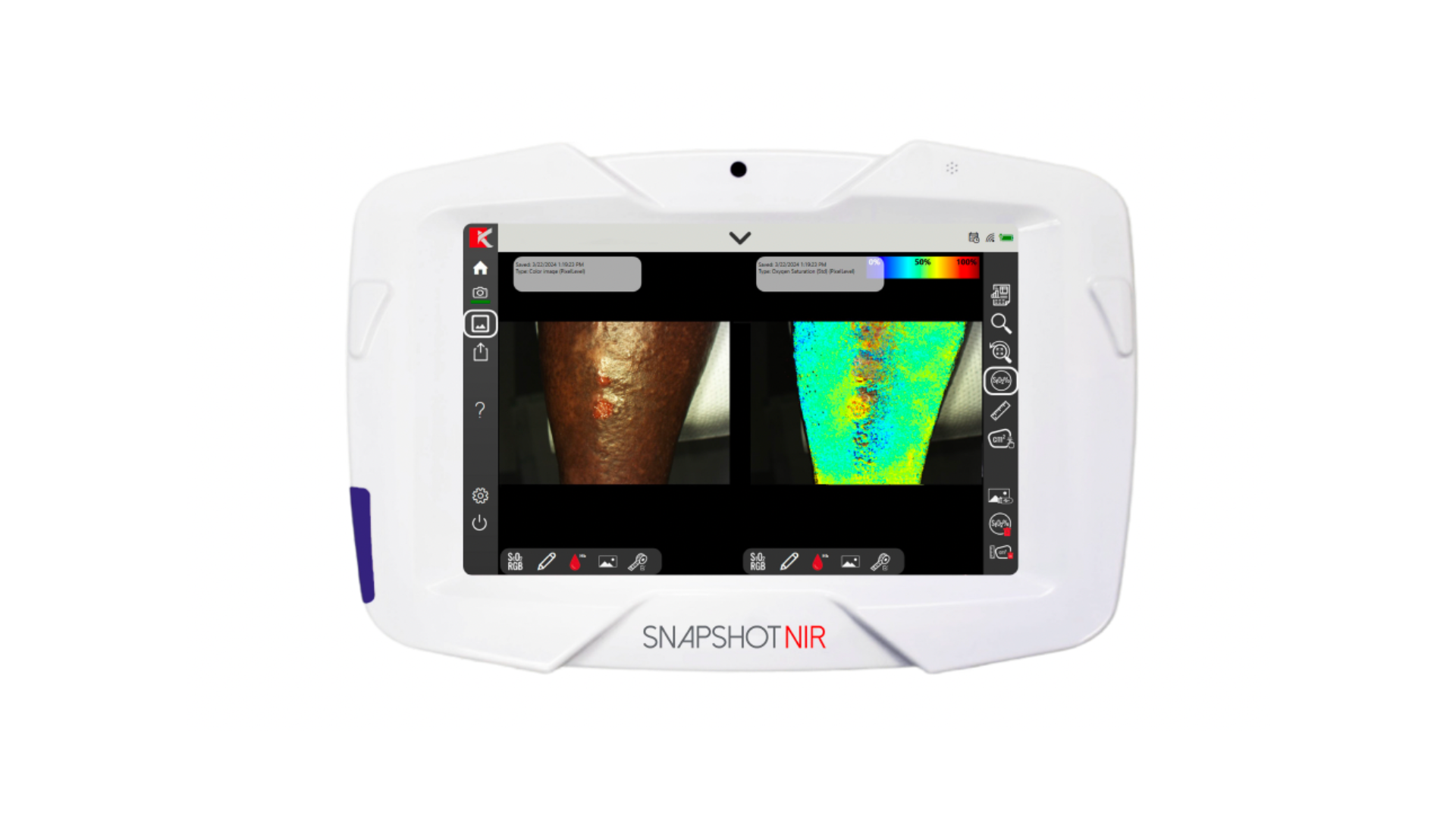
Kent Imaging is excited to announce the latest model of SnapshotNIR, KD205, with features that expand the application of the technology to include almost all skin tones, advancing tissue assessment for physicians and patients.
In addition to the same user-friendly interface and portability as past devices, the company shares that SnapshotNIR KD205 measures tissue oxygen saturation (StO2), oxyhemoglobin (oxy) and deoxyhemoglobin (deoxy) in individuals across most of the Fitzpatrick Skin Type (FST) scale, at any location on the body. This impressive step forward in the imaging industry will help support the provision of actionable tissue viability insights to more populations across the US.
SnapshotNIR is a non-invasive, non-contact, near-infrared spectroscopy (NIRS) imaging device that captures StO2 by measuring relative values of hemoglobin in microcirculation. Kent Imaging shares that the latest model update has increased sensitivity countering the light-scattering effect of melanin content in tissue. The device automatically accounts for different levels of melanin and adjusts to produce repeatable, objective imaging results on all areas of the body.
For the full press release and more information, click here.